Thermodynamic Test of Heating Water in a Microwave
Microwave ovens have become an indispensable part of modern kitchens, primarily due to their efficient way of heating food. The microwave oven uses electromagnetic radiation (waves) with a frequency of 2.45 GHz, corresponding to a wavelength of approximately 12 cm (the so-called microwaves), which vibrates the molecules of water, fats, and sugars in food. The vibration and mutual friction of the molecules generate heat directly within the food, thereby warming it. Microwaves are generated by a magnetron and directed into the cooking space. This allows heat to be created directly in the food, rather than by heating the air or containers as is the case with traditional cooking methods.
Difference Between Temperature and Heat
Temperature is a state physical quantity that describes whether a body is "warm" or "cold." It is measured with a thermometer, for example, in degrees Celsius [°C]. It is related to the magnitude of the vibration of molecules. Therefore, on a body with greater molecular vibration, we will measure a higher temperature value.
Heat is the amount of energy transferred between two bodies (at different temperatures). The unit of heat is the Joule [J]. If we want to express the amount of heat, we must express it in terms of the amount of energy supplied. For example, how much fuel or electrical energy we need to supply to heat a body.
Experiment with the Microwave
Since the microwave delivers energy for heating directly into the food and can also be set to a precise heating time, I was curious about how this relates to the resulting temperature to which the food is heated. By setting the correct parameters, it would be possible to heat the food to an exact temperature. A specific microwave oven, the SENCOR SMW5217SL, was used for testing, but with minor differences, this will also apply to other microwaves of similar construction.

tested microwave oven SENCOR SMW5217SL
Electrical Input/Output
The manufacturer specifies a rated input (consumption) of 1280W and microwave output of 800W. The actual consumption was measured at 1190W using a meter Sololight_DT27. When only the plate with the fan was spinning and the light was on, but the magnetron was not running, it was only 21W. Standby mode with the clock display on consumed only 3W. From this, the cost of electricity can be calculated.
Amount of Energy Over Time
In the test, 200ml of pure water in a glass cup was used. The heating started with cold tap water at a temperature of 17°C. After heating for 10 seconds, the temperature rose to 23°C, which is a difference of 23°C – 17°C = 6°C. I measured the temperature using a laboratory mercury thermometer and rounded the values to whole numbers.
To calculate the energy consumed, we will use the relationship:
Q = m × c × ΔT
- m is the mass of water (200ml of water ≈ 200g),
- c is the specific heat capacity of water (approximately 4.18J·g⁻¹·K⁻¹),
- ΔT is the temperature difference (23°C – 17°C = 6°C).
Substituting gives:
Q = 200g × 4.18J·g⁻¹·K⁻¹ × 6K = 5016J
Thus, approximately 5000 Joules of energy are consumed to heat 200ml of water from 17°C to 23°C.
Theoretical Calculation of Power for Temperature Increase:
The manufacturer-specified power of 800W means an energy of 800J per second. Therefore, in 10 seconds, we supply energy:
800W × 10s = 8000J
To calculate the temperature increase ΔT, we will use the relationship:
ΔT = Q / (m × c)
• Q = 8000J
• m is the mass of water (200ml ≈ 200g)
• c is the specific heat capacity of water (approximately 4.18J·g⁻¹·K⁻¹)
Substituting gives:
ΔT = 8000J / (200g × 4.18J·g⁻¹·K⁻¹) ≈ 9.6°C
The temperature of the water should ideally increase by approximately 9.6°C under conditions where all energy is utilized.
Extending Heating Time
I gradually changed the total heating time and measured the resulting temperature. Each heating session started with cold water from the tap. I used several identical cups so they could cool down between measurements. The water temperature was always 17°C at the beginning, and the time refers to the heating from the start to the end of the set duration. I displayed the measured data in a graph and fitted it with a linear curve. The slope of the curve indicates that for every 10 seconds, the temperature increases by approximately 6°C (this applies to 200ml of water at full 100% power).
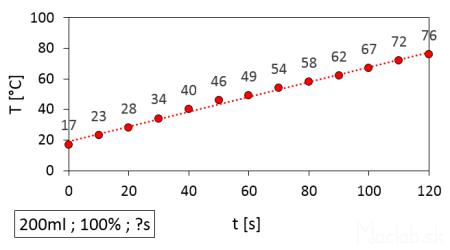
Heating 200ml of water in a microwave to a specific temperature depending on the set time.
Change in Water Volume
The above graph for heating applied to 200ml of water. However, we have already mentioned that the magnetron directs energy to heat the water, and there is always a certain amount of it. Analogously, the same amount of energy concentrated into a much smaller volume of water will result in a much higher temperature (for the same time).
In the next test, I heated different amounts of water for the same time of 20 seconds. The amount of water is related to its mass and volume. The same heating time is also related to delivering the same amount of energy.
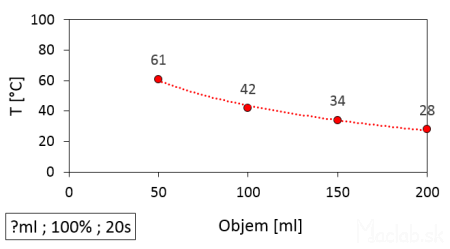
The temperature of water heated in a microwave depending on the heated volume for the same time of 20 seconds.
When changing mass while keeping the same amount of energy, we get an inversely proportional relationship. Theoretically, with a very large volume of water, the same amount of energy would not heat the water by even 1°C. Conversely, with a small volume of water, its temperature will rise to a high value. However, in reality, extreme reduction of the water volume also decreases the area that the microwave irradiates.
Effect of Power
The graph below shows the effect of setting different power levels on the achieved temperature. I always heated 200ml of water for the same duration of 120 seconds. The only thing that changed was the set power. A power setting of 0% corresponds to the initial temperature without heating. With a higher power setting, we achieve a higher water heating temperature.
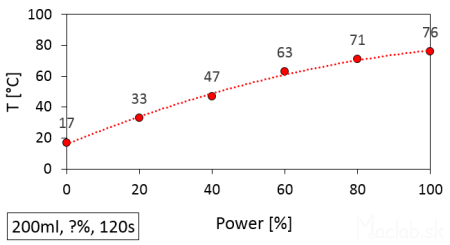
The temperature of water heated in a microwave depending on the set power.
Pulsed Power Change
The microwave allows for a change in heating power. However, this is not a change in power in the strict sense. At regular intervals, the magnetron (the source of microwaves) turns on and off. For example, if we set 50% power, the microwave turns the magnetron on for certain seconds and off for other seconds, thus delivering an average of only half the power (the magnetron is on for only half of the set time). The goal is to wait until the heat in the food is evenly distributed throughout the entire volume, so that the entire volume has approximately the same temperature at every point (i.e., heat from the warmer parts transfers to the cooler parts). However, repeated turning on and off of the magnetron can lead to reduced lifespan or earlier failure of the microwave.
Below are graphs showing the power progression over time. I measured the change in power over time to identify the start and end of the magnetron's switching. It can also be identified by sound; when the magnetron is off, the microwave makes noticeably less noise. This is a pulsed pattern. The sum of the durations of the individual pulses should correspond to a certain percentage of the full power.
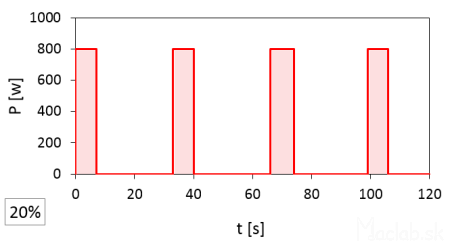
A set power of 20% has a pulse duration of 6 seconds.
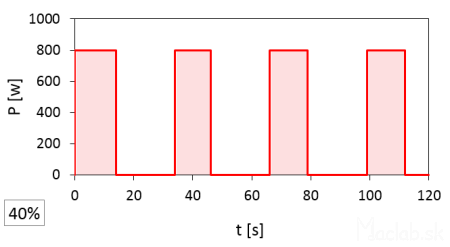
A set power of 40% has a pulse duration of 14 seconds.
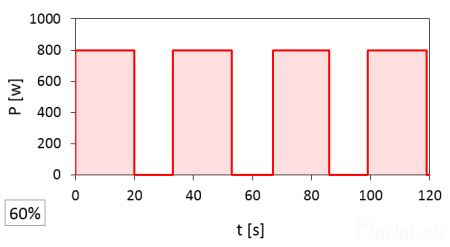
A set power of 60% has a pulse duration of 20 seconds.
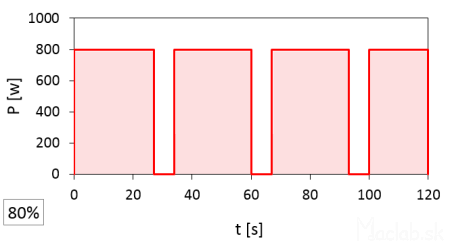
A set power of 80% has a pulse duration of 26 seconds.

A set power of 100% runs continuously.
At full 100% power, the magnetron is on for a full 120 seconds. At 20% power, the time width of one pulse is 6 seconds, repeating 4 times, so the total time is 6x4=24 seconds, which constitutes 20% of the total time at full power. I rounded the start and end times of the pulses and recorded them to whole seconds according to the stopwatch, so the actual values may vary slightly. It should also be noted that the interval between pulses is likely fixed, but after the set heating time has elapsed, the microwave stops even with an incomplete interval of the last pulse.
Conclusion
This was an exhaustive test that the manufacturer should have conducted. They likely tested it during the design of the microwave, but did not include it in the manual. Probably because the average person would not understand it anyway. In conclusion, if we have the same volume of a specific food with a specific initial temperature (for example, a cup of milk from the refrigerator), we can set a specific time to achieve heating to an exact temperature, so that the drink is neither cold nor hot. In reality, however, the type of food, its volume, and its initial temperature vary, and we have no choice but to test the temperature with our finger between short heating sessions.
What’s next?
How to calculate the cost of electricity